Nuvaxovid
88 experienced pain. Nuvaxovid is packaged as a ready-to-use liquid formulation in a vial containing ten doses.
Ema Chmp Recommends Cma For Novavax S Covid 19 Vaccine As Booster
Data från Australien pekar mot en ökad.
. Nuvaxovid contains a version of a protein found on the. COVID-19 Vaccine recombinant adjuvanted 2. This will enable us to start offering the Nuvaxovid.
1 day agoThe US company Novavax came up with another vaccine to fight the virus - Nuvaxovid. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022.
The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. 1 day agoPublicerad idag 0702. On December 20 2021 the.
Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. Horse serum of day 35 ie two weeks after the second dose pooled human serum from vaccinees of Moderna COVID-19 vaccine NRH-17846 and rabbit polyclonal anti-SARS-CoV-2. The vaccination regimen calls for two 05 ml doses 5 mcg antigen and 50 mcg Matrix.
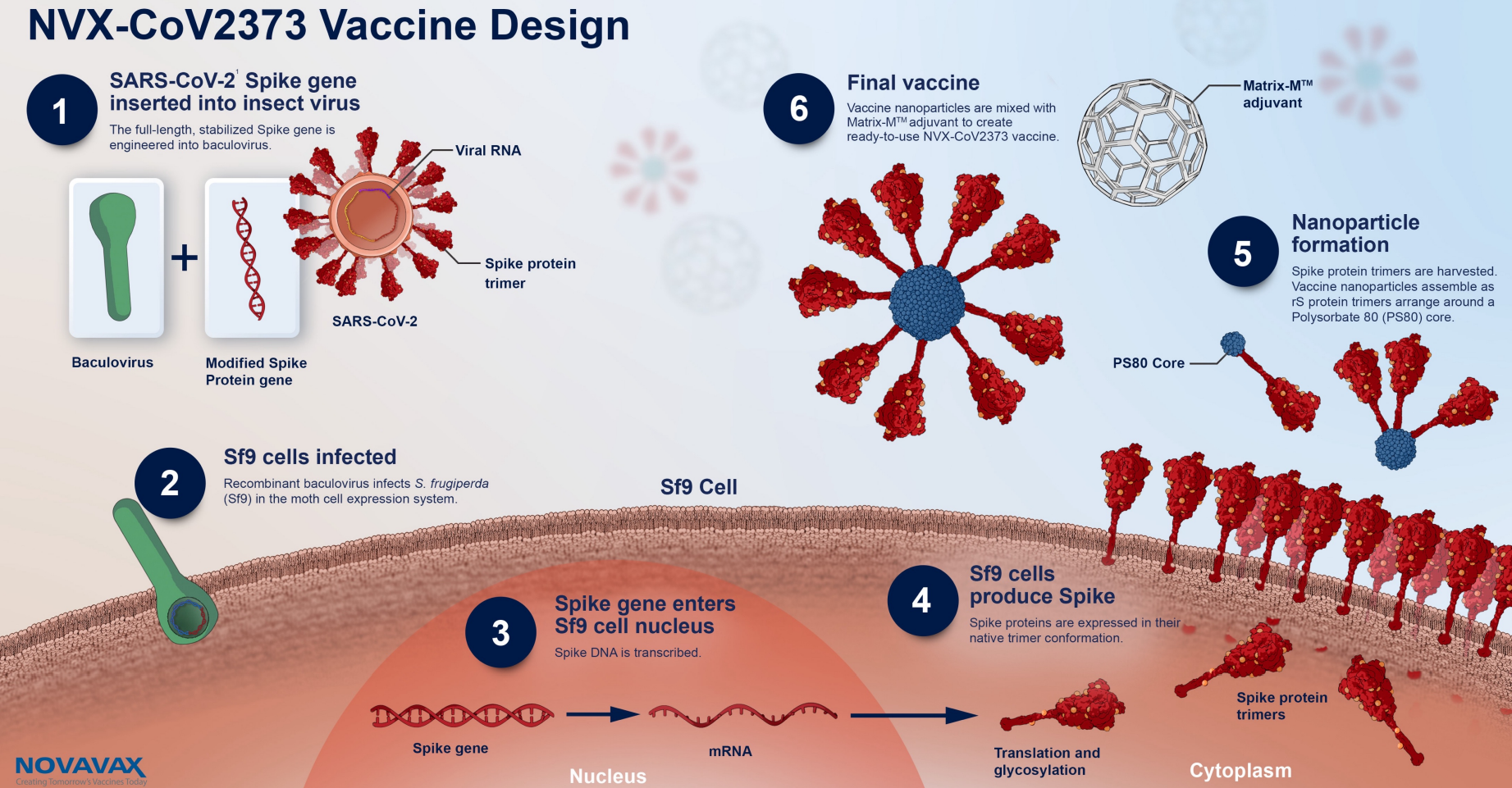
Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. Nuvaxovid-rokote sopii lähes kaikille aikuisille. The Summary of Product Characteristics is a description of a.
The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant. 16 fever including 14 severe cases. Like the Novavax vaccine side effects were more.
Qualitative and quantitative composition. Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30.
Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022. Nuvaxovid is the first protein-based COVID-19 vaccine granted. This vaccine is currently being used in Sweden and as of date a total of 7000.
The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19. After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further. Company Novavax should not be given to individuals.
About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach. Beslutet är temporärt och gäller från. Det eftersom att data från.
Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. 1 day agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Clinical trials showed that the vaccine has around 90 efficacy.
Nuvaxovid dispersion for injection. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. This is a multidose vial.
Name of the medicinal product. The first batch of Nuvaxovid is expected to arrive in. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten.
1 day agoBakgrunden till beslutet är signaler om ökad risk för hjärtmuskelinflammation myokardit och hjärtsäcksinflammation perikardit. Rokotteesta ei myöskään ole haittaa vaikka.

Novovax Provisional Approval For Over 12s The Canberra Times Canberra Act

Novavax Hopes Its Covid Shot Wins Over Fda Vaccine Holdouts The Boston Globe
Tga Investigates Possible Myocarditis Link To Nuvaxovid Ausdoc

Informaciya O Vakcine Protiv Covid 19 Nuvaxovid Novavax Australian Government Department Of Health And Aged Care
Fda To Authorize Novavax S Covid 19 Vaccine Politico
Switzerland Approves Novavax S Covid Vaccine For 12 18 Year Olds Swi Swissinfo Ch
Nuvaxovid Otazky A Odpovedi K Vakcine Od Spolecnosti Novavax Kurzy Cz

Vakcina Novavax Nuvaxovid Vaktsineeri Ee
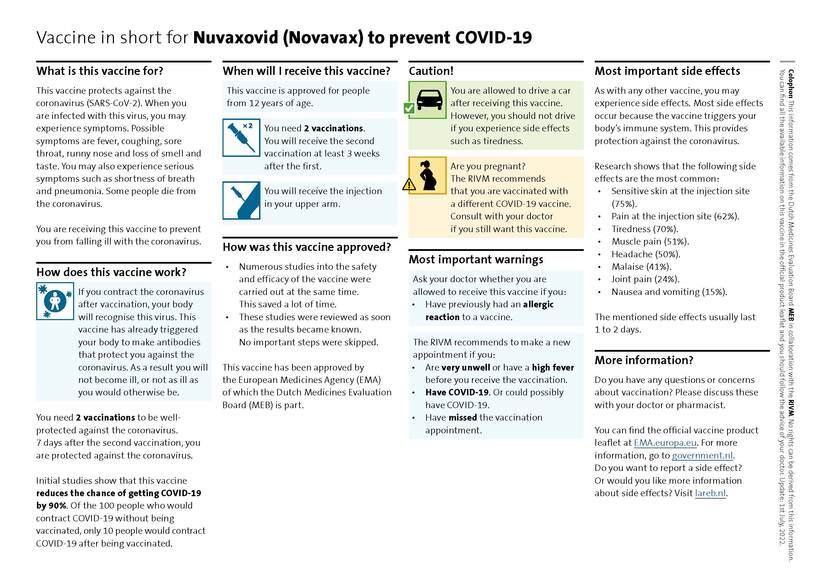
Vaccine In Short For Nuvaxovid Novavax Publication Medicines Evaluation Board
Racgp Tga Green Lights Nuvaxovid For 12 17 Year Olds
Dossier Coronavirus Sars Cov 2 And Covid 19 Suspected Adverse Reactions Reported In The First Three Months Since Start Of Vaccination With Nuvaxovid Paul Ehrlich Institut

Novaya Belkovaya Vakcina Nuvaxovid Pribyla V Estoniyu Vaktsineeri Ee
Nuvaxovid Covid 19 Vaccines And Treatments Portal

Is The Novavax Covid Vaccine Worth The Hype Medpage Today

Nuvaxovid Novavax S Covid 19 Vaccine Approved For 18y And Older The Immunisation Advisory Centre
More Than 18 000 Doses Of Nuvaxovid Covid 19 Vaccine Administered In Singapore 5 Reports Of Severe Reactions Cna
Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022

